Which of the Following Describes a Chemical Reaction
Melting an ice cube requires temperatures above. Atoms in substances react with each other describes a chemical reaction.
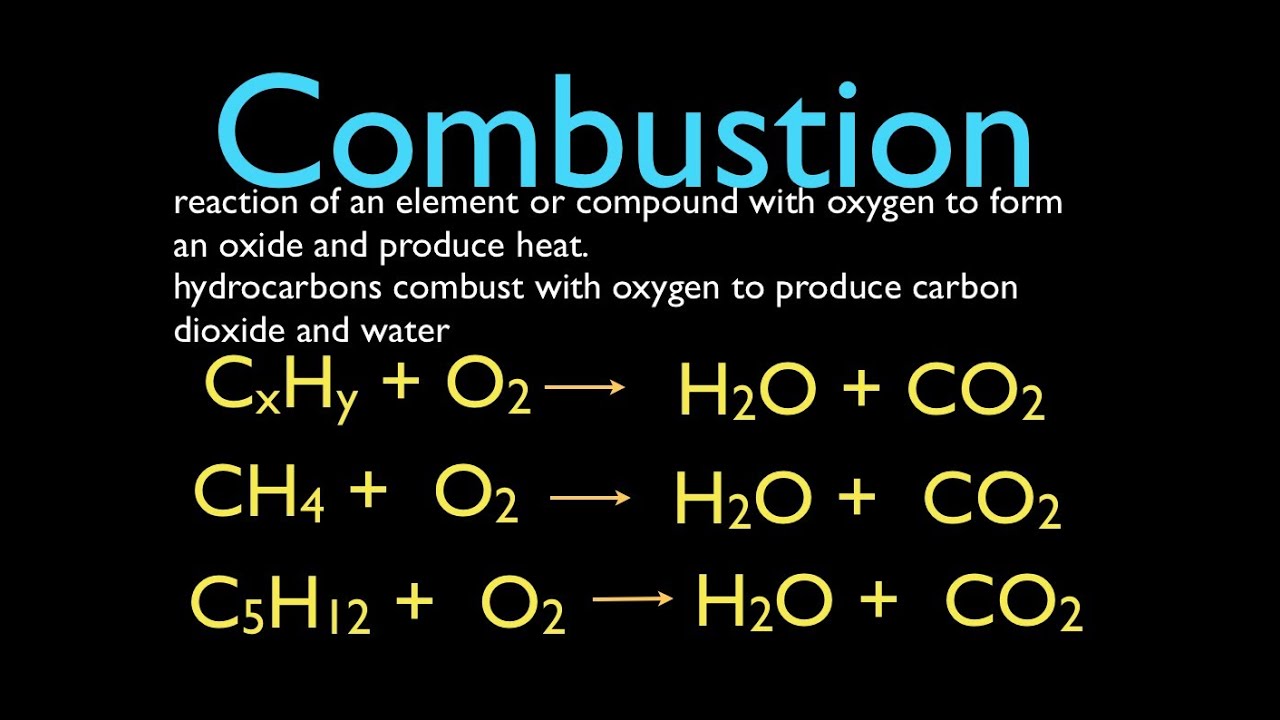
Combustion Reactions Chemical Reactions Chemical Equation Reactions
It is the volume in which the reactant particles can occupy.

. 2 C 2 H 5 OH l 2 CO 2 g C 6 H 12 O 6 C. 6 C g 6 H 2 g 3 O 2 g C 6 H 12 O 6 Solution. The substances that are formed B.
Chemical kinetics measures how. A chemical reaction is made up of two distinct parts which are reactants and products. D Metabolizing food supplies the.
NaNO3 MgSO4 - Na2SO4 Mg NO32 S. Which of the following statements correctly describe displacement reactions-displacement reactions are a type of oxidation-reduction reaction-displacement reactions always include a metal in a compound that is displaced by another metal in the elemental state. 6 C s 6 H 2 O l C 6 H 12 O 6 B.
Which of the following describes the entropy changes in gas reactions. The chemicals on the right side of a chemical equation. The reaction would be.
It is the amount of temperature needed to. Metabolizing food supplies the body with the energy required to heat ourselves and move. The reaction or process will always proceed to the left as written even if very slowly.
Chemical reaction a process in which one or more substances the reactants are converted to one or more different substances the products. Exposing water to cold temperatures results in freezing. The substances that are made C.
One branch of government can check the power of another branch of government. We can represent atoms by listing the number of protons neutrons and electrons-for example 2p 2n0 2e- for helium. A decrease in the number of gas molecules results in the entropy going to zero.
Congress must make sure that the national budget is balanced. Changing a carbon atom to a nitrogen atom by radioactive decay. Ons 2NaNO3 aq MgSO4 aq -- Na SO4 s Mg NO32 aq aluations 2NaNO3 aq MgSO4 aq - NaSO4 aq Mg NO32 s.
1 the light-independent reactions release energy and the light-dependent reactions require energy2the light-dependent reactions produce atp and nadph which are then used by the light. Which of the following best describes a chemical reaction system that has a positive total entropy change ΔS universe. Chemical kinetics is the study of reaction rates.
The formation of a covalent bond between two amino acids. The rates of the forward and reverse reactions are equal. Both endergonic and exergonic reactions require a small amount of energy to overcome an activation barrier.
B A natural gas burner can be used to heat water. Which of the following statements correctly describes any chemical reaction that has reached equilibrium. Increasing the temperature may speed up the reaction and it may affect the spontaneity of the reaction.
By the Law of Conservation of Energy we know that the total energy of a system must remain unchanged and that oftentimes a. Which of the following is a chemical reaction. C Melting an ice cube requires temperatures above freezing.
An increase in the number of gas molecules results in an increase of entropy. Which of the following comparisons or contrasts between endergonic and exergonic reactions is false. I think the correct answer from the choices listed above is option B.
6 C s 6 H 2 g 3 O 2 g C 6 H 12 O 6 E. Which of the following describes the reactants of a chemical reaction. A During photosynthesis plants harvest light energy in order to drive the synthesis of carbohydrates from CO2 and H2O.
Making a hydrogen bond between a water molecule and a sugar molecule. The products on the other hand refers to the new substance that is formed as a result of the reaction of the reactants. Chemical kinetics describes chemical reactions using collision theory.
Due to the absorption of energy when chemical bonds are broken and the release of energy when chemical bonds are formed chemical reactions almost always involve a change in energy between products and reactants. 6 C s 12 H g 6 O g C 6 H 12 O 6 D. Which of the following describes the principle of checks and balances A.
Which of the following chemical reactions describes the standard enthalpy of formation Δ H f for glucose C 6 H 12 O 6 s. It is the speed in which the reactants are converted into a product. O O O b.
Which of the following statements best describes the relationship between the light-dependent and light-independent reactions of photosynthesis. A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products. Substances are either chemical elements or compounds.
Which of the following statements describes an endothermic chemical reaction. Which of the following statements correctly describe s E1 reactions of alkyl halides RX. How is the government of Belarus different from those of other European countries.
The reactants refers to the starting material of the chemical process which react together under suitable conditions. Which of the following statements describes an endothermic chemical reaction. From the statement itself the word react is present this signifies a reaction.
Which of the following statements best describes the rate if a chemical reaction. The number of gas molecules involved in a. A decrease in the number of gas molecules results in an increase in entropy.
The melting of ice. The chemicals on the left side of a chemical equation D. View the full answer.
S Which of the following describes the chemical reaction after mixing aqueous sodium nitrate with magnesium sulfate solutions. A natural gas burner can be used to heat water.
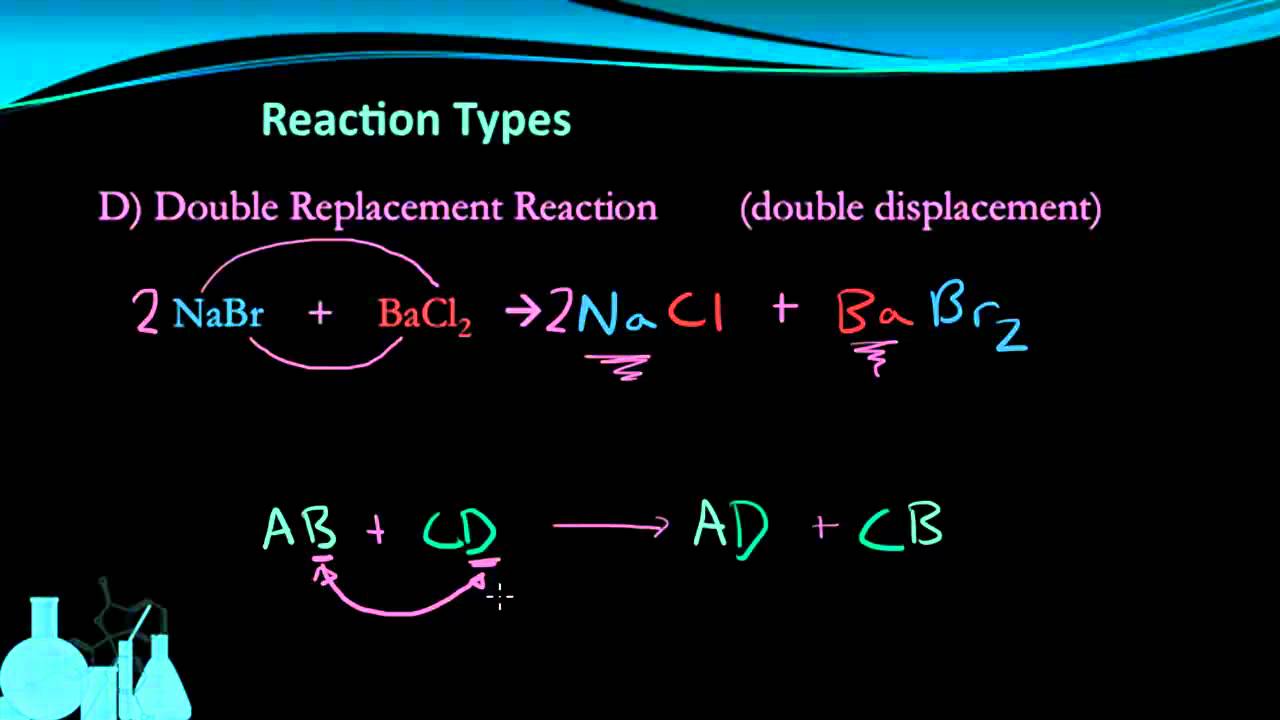
Chemistry Module 5 Types Of Chemical Reactions Http Www Youtube Com Watch V Aawccqb75d0 Single

Synthesis Physical Vs Chemical Properties Changes Physical Vs Chemical Properties Physics Teaching Chemistry

Scienceinvestigators Matter Chemical And Physical Changes Science Worksheets Physical Change
No comments for "Which of the Following Describes a Chemical Reaction"
Post a Comment